Top Links
Journal of Forensic Science & Criminology
ISSN: 2348-9804
Identification of Pyrrolidinophenone-type Designer Drugs by Gas Chromatography/Time-of-Flight Mass Spectrometry
Copyright: © 2014 Lopez-Avila V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Use of gas chromatography (GC) combined with time-of –flight mass spectrometry (TOFMS) with soft ionization generated with three different plasma gases (i.e., Xe, Kr, Ar) was evaluated for the identification of several pyrrolidinophenone-type designer drugs that are commercially available. Surprisingly, the mass spectra obtained with soft ionization exhibited only the immonium ions and lacked the molecular ions just like with 70 eV electron ionization. However, use of derivatization with 1-methyl-1-phenylhydrazine and soft ionization of the hydrazones with Ar plasma at 11.61/11.82 eV provides information that is helpful in structure elucidation. This paper focuses on the soft ionization of the hydrazones of 2,3-methylenedioxypyrovalerone (MDPV) and its deuterated analog containing eight deuterium atoms on the pyrrolidine ring, to establish a fragmentation pathway for the soft ionization of such compounds when using GC-TOFMS.
Keywords: Gas chromatography; Time-of -flight mass spectrometry, Soft-ionization; Pyrrolidinophenones; Derivatization with 1-methyl-1-phenylhydrazine
Pyrrolidinophenones are synthetic cathinones with new chemical functionalities that have managed to avoid regulatory oversight until they were discovered (i.e., MDPV in 2007) and legislative measures were implemented against such dangerous compounds [1]. Several analytical techniques like GC ion trap or quadruple MS with electron ionization (EI) or chemical ionization (CI) [2-6], and high performance liquid chromatography (HPLC) MS [7] as well as nuclear magnetic resonance (NMR) spectroscopy [2,5] have been used to identify such compounds. However, NMR spectroscopy is expensive because it may require not only onedimensional but two-dimensional 1H- and 13C-spectroscopy [5], furthermore it requires microgram quantities of the analyte for identification.
When using GC-MS with EI, the unpaired electron on the N atom induces an α-cleavage of the benzyl bond leading to the formation of an abundant immonium ion at m/z 126 for MDPV [2-4,6], and m/z 112 for MDPBP [5]. The EI mass spectra of a series of homologs and regioisomers of MDVP also yield only the immonium ion [6]. Therefore, pyrrolidinophenones are difficult to identify because their EI mass spectra lack the molecular ion and yield only an abundant fragment ion, the immonium ion (i.e. C6H12N at m/z 98.0964 for α-PPP, MPPP, MOPPP, and MDPPP ; C7H14N at m/z 112.1121 for α-PBP, 2-, 3- or 4-MPBP, and MDPBP; C8H16N at m/z 126.1277 for α-PVP, MDPV, and naphyrone; and C9H18N at m/z 140.1434 for MPHP), which was measured by high-resolution mass in this study (see Table 1). For this reason, chemical ionization (CI) is always used in conjunction with EI. Although CI provides information on the molecular ion, it does not provide enough information on the functional groups on the phenyl ring or the alkyl moiety attached to the Cα atom. However, product ion mass spectrometry of the immonium ions was used successfully to identify the alkyl-amino moiety [2].
This study focused on the identification of pyrrolidinophenone-type designer drugs using GC-TOFMS with soft ionization, only to discover that even despite the softest ionization with Xe plasma at 8.44 eV, there were no molecular ions in the spectra and the only ions were the immonium ions. Therefore, derivatization of the carbonyl group in combination with soft ionization and highresolution mass spectrometry was undertaken to investigate whether additional fragment ions obtained by soft ionization with a plasma gas could be helpful in structure elucidation.
MDPV and MDPV-D8 (both as free bases) were from Cerilliant (Round Rock, TX, USA). Spectroscopic-grade ethyl acetate and absolute ethanol (ACS reagent, >99.5% purity, 200 proof) were from J.T. Baker (Phillipsburg, NJ, USA) and EMD (Gibbstown, NJ, USA), respectively. 1- Methyl-1-phenylhydrazine (97%) was from Sigma-Aldrich (St. Louis, MO). Research Plus Grade Xe and Ultra High Purity Kr were purchased from Scott Specialty Gases (Plumsteadville, PA, USA) and Ultra High Purity Ar was from Airgas USA, LLC (Long Beach, CA, USA).
The derivatization was carried out in a closed conical-shape 1-mL vial (Supelco, Bellafonte, PA, USA), at 65 °C for one hour, using 100 μL of ethanol, 500 μg of MDVP or MDVP-D8, 40 μL neat methylphenylhydrazine and 10 μL acetic acid. After one hour, the test mixture was evaporated to dryness using pure nitrogen and was reconstituted with 1 mL of ethyl acetate. The derivatization yield has not been optimized in this study.
The exact mass measurements were performed with a research TOF mass spectrometer that was equipped with a prototype soft ionization source, operated in positive mode, and was interfaced to an Agilent 7890 GC (Agilent Technologies, Santa Clara, CA, USA). The TOF mass spectrometer was a modified Agilent 6220 Accurate-Mass TOF LC/MS system with a proprietary flight tube sealed in a vacuum insulated shell, in orthogonal configuration, a dual stage ion mirror that gives a flight path of 2 m, and an analogto-digital (ADC) detector with a 4 GHz sampling rate. Spectral data were acquired at 5 Hz acquisition rate and the mass range for data acquisition was 42 to 600 u. The mass axis was calibrated daily with perfluorotributylamine (PFTBA), which was delivered to the ionization source via a calibration valve, using Ar plasma (ionization energy of PFTBA is 11.3-11.7 eV). The resolution of the TOF mass spectrometer was at least 10,000 (full width at half height, FWHM) as measured with octafluoronaphthalene at m/z 271.9867. Samples were introduced via a 30 m x 0.25 mm id x 0.25 μm film thickness HP-5MS (5% phenyl 95% methylsiloxane) capillary column from Agilent Technologies. The oven temperature was programmed from 50 °C to 245 °C at 35 °C/min and then to a final temperature of 300 °C at 15 °C/min, where it was held for 3 min. Helium was used as carrier gas at a constant flow rate of 1.2 mL/min. The injector temperature was 250 °C, the source temperature was 175 °C (not optimized, although it can be heated up to 275 °C), and the GC-MS transfer line temperature was 300 °C. The injector, fitted with a 4-mm id double tapered liner (Agilent Technologies part 5181-3315), was set in splitless mode for 2 min after the injection (purge flow was 50 mL/min). Data processing was performed using the MassHunter Qualitative Analysis software (Agilent Technologies, version B.05.00) and chemical formulas were obtained using the Qual Formula Calculator algorithm incorporated in the MassHunter software.
The soft ionization source schematic is shown elsewhere [8]. Vacuum ultraviolet (VUV) light is produced by resonant microwave devices designed to ignite and sustain a plasma discharge at reduced pressures. These miniaturized flow resonance lamps typically operate on a few watts of power near 2.5 GHz at flow rates below 10 mL/min. The analyte coming from the GC column flows through a channel in the plenum and is exposed to VUV light through an orifice. The microplasmas are offset from the sample ionization chamber, allowing metastable atoms to be dispersed and thus reducing their interaction with the analyte. Electrostatic deflectors situated between the outputs of the plasma devices and entrances to the ionization chamber prevent plasma ions and electrons from entering the ionization zone and interacting with the analyte. The sample ions are extracted and formed into a beam using a custom ion source based on the design of the Agilent Technologies EI source.
Figure 1 shows the soft ionization spectra of MDPV using Xe (8.44 eV), Kr (10.02 and 10.63 eV) and Ar (11.61and 11.82 eV) indicating that regardless of how soft the ionization is, the most abundant ion is the immonium ion C8H16N at m/z 126.1277. The immonium ions that were found in the mass spectra of the other 12 pyrrolidinophenones are shown in Table 1. Although the highresolution data provided the accurate mass of these immonium ions, from which a formula could be derived for this fragment ion, lack of molecular ions or any other fragment ions in the mass spectra of the pyrrolidinophenones makes the identification of these compounds difficult even when using high-resolution mass spectrometry. Therefore, other approaches need to be considered when the identification of these compounds is done by mass spectrometry.
As the pyrrolidinophenone-type designer drugs contain a tertiary amine and a carbonyl group, the only moiety that can be derivatized in this case is the carbonyl group. A review article on the most important derivatizing agents for ketones [9] indicates that derivatization with hydrazine-type compounds is quite common in HPLC as compared to GC, because the resulting hydrazones are well suitable for UV and fluorescence detection. Furthermore, when using GC, the large amount of nitrogen in the hydrazone derivative makes the application of a nitrogen selective detector quite appealing, but it does require reference standards for retention time matching. To the best of my knowledge, there is no other publication on the derivatization of MDPV and MDPV-D8 with 1-methyl-1-phenylhydrazine and the identification of the respective hydrazones by GC-TOFMS with soft ionization.
Figure 2 shows the Ar soft ionization spectra of the corresponding hydrazones of MDPV and MDPV-D8. The most abundant ions in the spectrum of MDPV are the ions at m/z 70.0651, m/z 124.1121, m/z 204.1019, and m/z 273.1598, and in the spectrum of MDPV-D8 the ions at m/z 78.1153, m/z 132.1623, m/z 205.1082, and m/z 281.2100. It is interesting to note that most major ions in the spectrum of MDPV-D8 are shifted by eight atomic mass units (u) because of the eight D atoms, and one set of ions is shifted only by 1 u (i.e., m/z 204.1019 and m/z 205.1082) corresponding to only one D atom. There is another set of less abundant fragment ions at m/z 258.1363/266.1643 and m/z 230.1050/238.1552 that are also shifted by 8 u that would correspond to eight D atoms. The molecular ions of these hydrazones were not present at all, despite the fact that 1-methyl-1-phenyl hydrazones of acetone and propiophenone yield molecular ions with relative abundances of 100% and 82%, respectively. Nonetheless, the mass spectra of the hydrazones contained enough fragment ions, and from which a molecular formula can be generated. The proposed fragmentation pathway for the hydrazones of MDPV and MDPV-D8 is shown in Figures 3 and 4, respectively, and it is described below.
Elimination of 1-methyl-1-phenyl amine (C7H8N) radical from the molecular ions of both hydrazones yields the even-electron ions at m/z 273.1598 and m/z 281.2100 corresponding to C16H21N2O2 and C16H13D8N2O2, respectively. Further elimination of methyl and propyl radicals from the ion at m/z 273.1598 of MDPV yields the odd-electron ions at m/z 258.1363 and m/z 230.1050. As expected, elimination of methyl and propyl radicals from the ion at m/z 281.2100 of the deuterated hydrazone yields the oddelectron ions at m/z 266.1865 and m/z 238.1552, containing eight D atoms. The fragment ions at m/z 258.1363 and 230.1050 with relative intensities of 20-40% help identify the alkyl moieties attached to the Cα atom. The relative intensities of these ions are much more abundant by soft ionization than the 70 eV EI as reported by Westphal et al. [2].
The abundant ions at m/z 204.1019 and m/z 205.1082, which resulted from the elimination of the pyrrolidine radical (C4H7N) or its deuterated analog C4D7N from the even-electron fragment ions C16H21N2O2 or C16H13D8N2O2, respectively, confirm the transfer of an H or a D radical from the pyrrolidine ring to the Cα atom. Such elimination was not reported in MS, but sigma cleavage of the immonium ion at m/z 126 following the H transfer, which gave an abundant radical cation at m/z 69, was reported in the product ion spectra of MDPV [2].
Soft ionization led to the formation of an abundant ion at m/z 70.0651 for MDPV and m/z 78.1153 for MDPV-D8, which are even electron ions formed by breaking of the Cα-N bond. This is possible probably because the positive charge in the molecular ion of MDPV can be on any of the three N atoms. Formation of the abundant ion at m/z 124.1121 corresponding to the conjugated iminium cation is only possible if the positive charge is on N atom in the pyrrolidine ring. Subsequent elimination of C4H6 from the iminium cation led to formation of C4H8N ion at m/z 70.0651. Porter [10] reported a similar finding on pyrrolidine enamines in which a loss of the N-substituent led to the formation of the C4H8N ion.
To test the fragmentation scheme proposed above, pyrovalerone, which is similar to MDPV but has a methyl-phenyl ring instead of the 2,3-methylenedioxyphenyl ring ,was derivatized with 1-methyl-1-phenylhydrazine and the mass spectrum of the resulting hydrazone is shown in Figure 5. As expected, a loss of C7H8N from the molecular ion would lead to formation of the ion C16H23N2 at m/z 243.1856 and subsequent loss of methyl and ethyl radicals would lead to formation of ions C15H20N2 and C14H18N2 at m/z 228.1687 and m/z 214.1706, respectively. The abundant ion at m/z 124.0966 corresponds to C8H14N and the ion at m/z 69.0573 is C4H7N. The inductive effect of the methyl group attached to the phenyl ring appears to account for the presence of the C12H15 ion at m/z 159.1168 and ion C4H7N at m/z 69.0573 (100% relative abundance). The fragmentation of the pyrrolidinophenones containing the alkyl-substituted phenyl ring would need to be validated with deuterated standards that have D at the alkyl groups on the phenyl ring, but for the most part the soft ionization of 1-methyl-1-phenyl hydrazone of pyrovalerone follows the fragmentation scheme proposed here.
Derivatization of pyrrolidinophenones with 1-methyl-1-phenylhydrazine and soft ionization is needed because the conventional EI spectra lack molecular ion information and the only fragment ion present in the EI spectra are the immonium ions. Although the soft ionization spectra shown here still lack molecular ion information, there are enough fragment ions in the spectra of the hydrazones that can be used to piece together the intact molecule.
The author would like to acknowledge James Cooley (formerly with Agilent Technologies) and Randall Urdahl for the design of early prototypes of the soft ionization source, and George Yefchak and August Hidalgo for the ionization source control software. The plasma chip was fabricated by Martin Guth, formerly with Agilent Technologies.
 |
| Figure 1: Xe-, Kr-, and Ar-soft ionization spectra of the underivatized MDPV obtained by GC-TOFMS. |
 |
| Figure 2: Ar-soft ionization spectra of the 1-methyl-1-phenyl hydrazones of MDPV (lower) and MDPV-D8 (upper) |
 |
| Figure 3: Proposed fragmentation pathway for the 1-methyl-1-phenylhydrazone of MDPV using soft ionization and GC-TOFMS. |
 |
| Figure 4: Proposed fragmentation pathway for the 1-methyl-1-phenylhydrazone of MDPV-D8 using soft ionization and GC-TOFMS. |
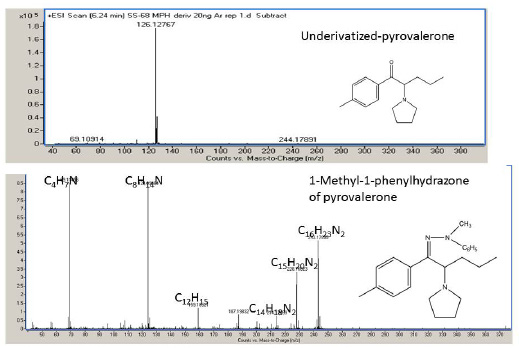 |
| Figure 5: Ar-soft ionization spectra of the underivatized pyrovalerone (upper) and of the 1-methyl-1-phenylhydrazone of pyrovalerone (lower) |
| Abbreviation | Chemical name | Formula (molecular ion) | Calc. m/z (molecular ion) | Calc. m/z (immonium ion ) | Formula (immonium ion) |
|---|---|---|---|---|---|
| α-PPP | 1-phenyl-2-(1-pyrrolidinyl)-1-propanone | C13H17NO | 203.1305 | 98.0964 | C6H12N |
| α-PBP | 1-phenyl-2-(1-pyrrolidinyl)-1-butanone | C14H19NO | 217.1461 | 112.1121 | C7H14N |
| MPPP | 1-(p-tolyl)-2-(1-pyrrolidinyl)-1-propanone | C14H19NO | 217.1461 | 98.0964 | C6H12N |
| 2-MPBP | 1-(o-tolyl)-2-(1-pyrrolidinyl)-1-butanone | C15H21NO | 231.1618 | 112.1121 | C7H14N |
| 3-MPBP | 1-(m-tolyl)-2-(1-pyrrolidinyl)-1-butanone | C15H21NO | 231.1618 | 112.1121 | C7H14N |
| 4-MPBP | 1-(p-tolyl)-2-(1-pyrrolidinyl)-1-butanone | C15H21NO | 231.1618 | 112.1121 | C7H14N |
| α-PVP | 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone | C15H21NO | 231.1618 | 126.1277 | C8H16N |
| MOPPP | 1-(4-methoxyphenyl)-2-(1-pyrrolidinyl)-1-propanone | C14H19NO2 | 233.1410 | 98.0964 | C6H12N |
| MDPPP | 1-(1,3-benzodioxol-5-yl)-2-(1-pyrrolidinyl)-1-propanone | C14H17NO3 | 247.1203 | 98.0964 | C6H12N |
| MPHP | 1-(p-tolyl)-2-(1-pyrrolidinyl)-1-hexanone | C17H25NO | 259.1931 | 140.1434 | C9H18N |
| MDPBP | 1-(1,3-benzodioxol-5-yl)-2-(1-pyrrolidinyl)-1-butanone | C15H19NO3 | 261.1359 | 112.1121 | C7H14N |
| MDPV | 1-(1,3-benzodioxol-5-yl)-2-(1-pyrrolidinyl)-1-pentanone | C16H21NO3 | 275.1516 | 126.1277 | C8H16N |
| Naphyrone | 1-(2-naphthalenyl)-2-(1-pyrrolidinyl)-1-pentanone | C18H23NO | 281.1774 | 126.1277 | C8H16N |
| Table 1: Chemical names of the test compounds, their molecular formulas and mono isotopic masses of molecular ions, and the formulas and mono isotopic masses of the immonium ions | |||||














